NEWYou can now listen to Fox News articles!
Sen. Josh Hawley, R-Mo., accused the Food and Drug Administration (FDA) of endangering women’s health, saying the agency approved another chemical abortion drug without the thorough safety review it had promised.
Hawley argued the move shows both regulatory failure and the influence of a company that refuses to define “woman” in its materials.
“This is shocking. FDA has just approved ANOTHER chemical abortion drug, when the evidence shows chemical abortion drugs are dangerous and even deadly for the mother. And of course 100% lethal to the child,” he wrote on X on Thursday afternoon.
“FDA had promised to do a top-to-bottom safety review of the chemical abortion drug, but instead they’ve just greenlighted new versions of it for distribution. I have lost confidence in the leadership at FDA.”
PRO-LIFE GROUP URGES SENATE TO PRESS RFK JR. ON ABORTION PILL SAFETY, DEMAND SAFEGUARDS RETURN

Sen. Josh Hawley, R-Mo., accused the Food and Drug Administration of endangering women’s health after it approved another chemical abortion drug without what he said was a promised full safety review. (Bill Clark/CQ-Roll Call, Inc via Getty Images)
Evita Solutions describes its mission as one to “normalize abortion” and make it “accessible to all.” On its website, the company says it “believes that all people should have access to safe, affordable, high-quality, effective, and compassionate abortion care, regardless of their race, sex, gender, age, sexuality, income, or where they live.
“We know that you can make the best choice for your body.”
According to the FDA, Evita received approval in a Sept. 30 letter obtained by Reuters.
In an interview with Fox News Digital, Hawley said the FDA’s decision was even more troubling given its promised safety review has barely begun.
“I just, I can’t figure out what’s happening at the FDA. I’m totally baffled by it,” Hawley said.
Fox News Digital has reached out to the FDA and Evita Solutions for comment on the matter.
FDA CHIEF HAS NO ‘PLANS’ FOR ABORTION PILL POLICY CHANGES BUT CONTINUES SAFETY REVIEW
In another post, Hawley blasted the FDA for partnering with a company that “doesn’t even believe there is such a thing as a ‘woman.’”
Evita Solutions now joins GenBioPro in producing the generic version of mifepristone, the abortion pill originally made by Danco Laboratories. Mifepristone blocks progesterone, a hormone needed to sustain pregnancy, and is followed by misoprostol to complete the process.
The approval comes as abortion drugs face mounting opposition from conservative lawmakers, religious organizations and pro-life groups.
MORE THAN 20 GOP ATTORNEYS GENERAL CALL ON RFK JR, FDA TO REINSTATE SAFEGUARDS FOR ABORTION DRUGS
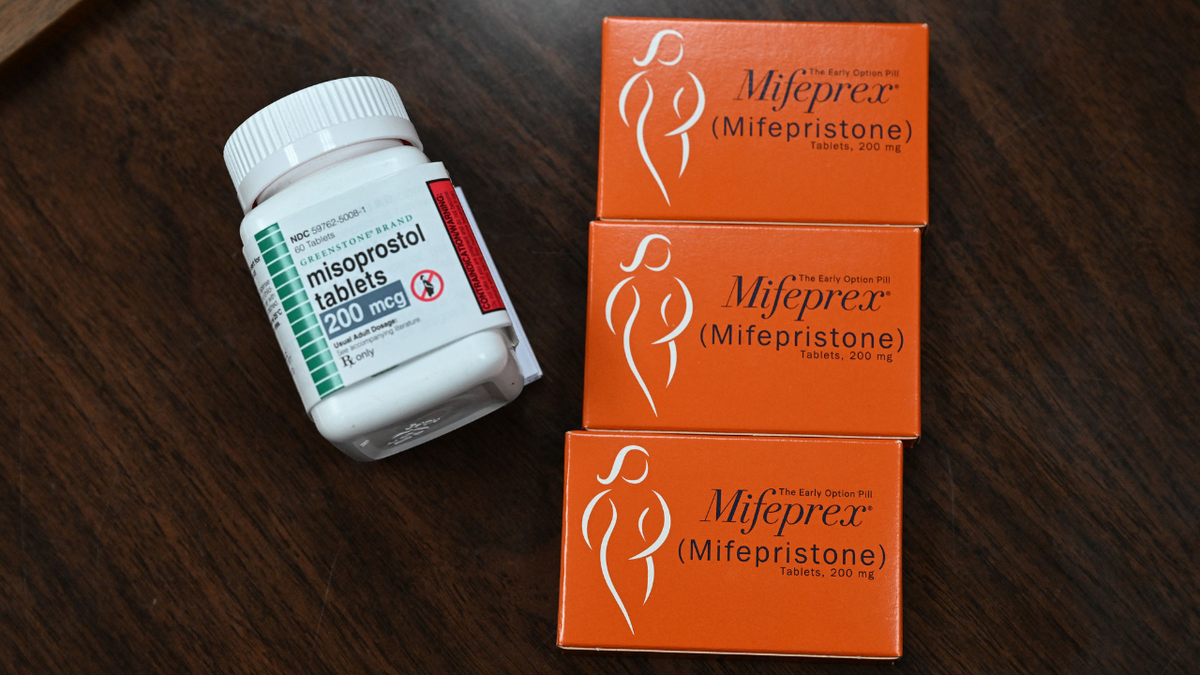
Misoprostol, left, and mifepristone, the two drugs used in a medication abortion. (Robyn Bech/AFP via Getty Images)
Religious groups like Inspire Investing and Alliance Defending Freedom have campaigned against the drug, while the Restoration of America Foundation (ROAF) has pressed lawmakers for accountability.
Last month, ROAF called on the Senate Finance Committee to hold Health and Human Services Secretary Robert F. Kennedy Jr. accountable at a hearing, demanding answers about the removal of safety protocols for the abortion pill mifepristone.
In a letter obtained by Fox News Digital, ROAF warned that the rollback leaves women more vulnerable and shifts costs to taxpayers. The group said the Biden-era changes endanger women by allowing abortion pills to be prescribed via telehealth and sent through the mail.
Hawley said the FDA should restore the safeguards put in place under the Trump administration.
“What needs to happen is the FDA needs to get in line with the president’s policy and put back into place the safety regulations President Trump had. Ditch the Biden approach and go back to President Trump’s approach,” Hawley said.
CLICK HERE TO DOWNLOAD THE FOX NEWS APP
Under the Biden administration, the FDA for the first time allowed telehealth prescribing and mail-order delivery of abortion pills. Previously, the agency required mifepristone to be dispensed in person to screen for complications such as ectopic pregnancy.
Fox News Digital’s Jasmine Baehr and Reuters contributed to this report.


